- New
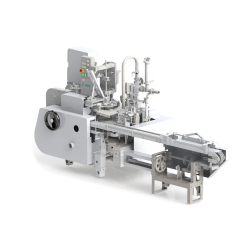
GEA Aseptic Blow Fill System ABF 2.0
The ABF 2.0 is the latest evolution of the world’s first rotary aseptic blow molding machine with an integrated aseptic filler and capper. It’s designed to sterilize the preform with hydrogen peroxide when leaving the oven, then blow the preforms with sterile air in an aseptic environment and maintain this sterility throughout the filling and capping process.
Description
The concept
The preforms are sterilized as soon as they leave the oven, where they are heated to optimize their thermal profiling and to minimize energy consumption and the use of the sterilizing agent. The internal and external surfaces of the preforms are treated with vaporized hydrogen peroxide (VHP) using a robust, controlled and condensation-free process to minimize residue values and achieve a 6 log decontamination performance.
After the sterilization process, the preforms enter a pre-sterilized microbiological isolator – where the aseptic blowing wheel is located – and are blown with sterile air supplied through a filtered circuit that includes a dedicated VHP sterilization system.
The fresh-blown sterile bottles are transferred by the neck from the aseptic blower to the aseptic filling module without leaving the sterile zone. The aseptic filler is enclosed in the same microbiological isolator, in which sterility is maintained during production with an overpressure of class 100 sterile air. As well as the filler module, the capper module in the ABF 2.0 system is specifically designed for aseptic applications and completely enclosed within the microbiological isolator.
Benefits:
- Full automated sterilization of the blowing, filling and capping modules, resulting in an effective, repeatable and automated process, needing no operator intervention
- Low chemical and no water consumption during production
- Minimum H2O2 residuals in the container (
- Limited downtime (3 hours cleaning and sterilization cycles) for product changeovers
- Microbiological validation according to specific protocols for sensitive beverage industry
- PoC approved, being based on ABF 1.2 FDA approved architecture
Performance
The versatile ABF 2.0 aseptic production system can process extremely sensitive, high and low acid, still or carbonated beverages, with or without particles. It can deliver a 6 log reduction for both cap and preform decontamination in a single sterilization phase and offers continuous aseptic production runs of 165 hours without stoppages or intermediate cleaning.
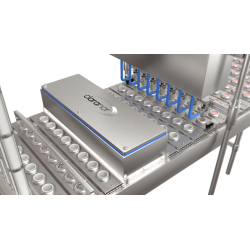
H2O2 technology for cap and foil sterilization
Similar to the preform sterilization system, the ABF 2.0 cap sterilization module – Sterilcap VHP R – also uses VHP at the correct concentration and temperature. The specially adapted design, including a rotary cap buffer that guarantees the necessary redundancy required by the blow-fill technology, ensures that all the cap surfaces are exposed, preventing any ‘shadow’ areas and avoiding the risk of cap deformation.
The cap sterilization module can be used to treat both sport and flat caps. Offering rapid changeovers without any mechanical intervention and without losing sterility, the ABF 2.0 solution offers maximum flexibility when treating aluminum foil closures with Sterilfoil VHP L technology.
220 other products in the same category:
- New
- New
DABL series automatic aseptic plastic bottle filling and capping machine
- New
- New
- New
- New
LQ-RCGF Washing-filling-capping 3 In 1 Machine For Juice
- New
- New
- New
PSM-06 - Stick and Tetrahedron Pack Powder Filling Machine
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
Vertical form-fill and seal machine of the “Economy” series
- New
- New
- New
- New
- New
Canfillmatic TESTAROSSA Filling lines for carbonated liquids
- New
- New
- New
- New
Filling and seaming groups for aluminium and tin plate cans
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
- New
Highly corrosive product rotary pressure overflow filler model RF48-18-BL
- New
TPM 1000 fully automatic thermoforming packaging machine
- New
- New
- New
- New
- New
- New
Pneumatic piston filling dispenser for liquids 300 - 2500 ml
- New
- New
- New
- New
- New
- New
GF series automatic plastic bottle filling and capping production line
- New

 English
English
 Deutsch
Deutsch
 Français
Français
 Español
Español
 Italiano
Italiano
 Português PT
Português PT